Anatomy illustrations from text
Figure A-2
Pancreas. Note that the pancreatic ducts and the bile duct (from the liver) both empty into the duodenum in the same vicinity.
Figure A-3
Cross section of the stomach. Food enters the stomach from the esophagus, and empties into the duodenum. The cardiac and pyloric sphincters function to keep food in the stomach while that part of digestion takes place.
Figure A-5
The liver. Bile is collected from throughout the liver via the main hepatic duct and its smaller branches, and then either moved via the cystic duct and stored in the gall bladder until needed or passed directly into the duodenum via the common bile duct.
Figure A-7
Schematic diagram of the circulatory system. Blood is pumped from the right atrium into the right ventricle, and from there to the lungs, where excess carbon dioxide is removed and fresh oxygen acquired. Returning from the lungs, the blood passes through the left atrium and then the left ventricle to be distributed to the rest of the body, including the heart itself. Returning from the body, blood is now back at the right atrium.
Figure A-8
Cross section of the heart. The structures within the ventricles are the chordae tendinae, which serve to keep the tricuspid and mitral valves from collapsing back into the heart during pumping contractions of the ventricles.
Figure A-9
The valves of the heart. The mitral valve is also known as the bicuspid valve; the aortic and pulmonary valves are sometimes referred to as the semilunar valves.
Figure A-10
Exterior views of the heart showing the coronary arteries, plus a schematic cross section diagram of a coronary artery showing how arteriosclerosis can block the artery, reducing blood flow o the heart muscles.
Figure A-13
Schematic diagram of bronchioles and alveoli within the lungs. Blood flowing form the heart moves through smaller and smaller branches until it reaches the capillary nets that surround the alveoli. Oxygen is taken into the blood from inhaled air within the alveoli, and carbon dioxide is released from the blood into the alveoli; it is then expelled from the body during exhalation.
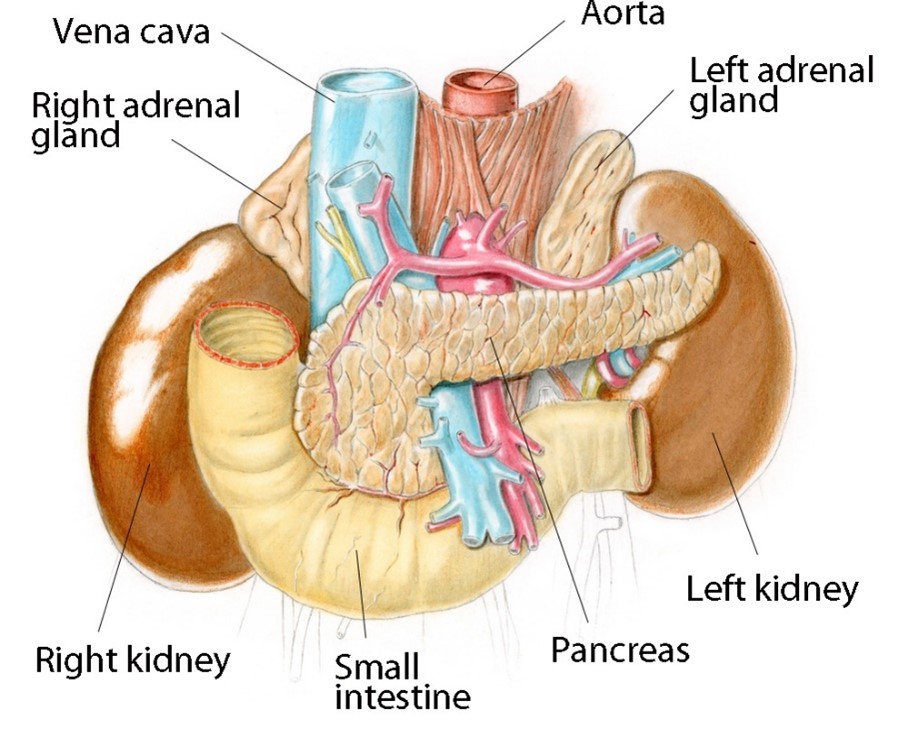
Figure A-15
The kidneys and adrenal (or suprarenal) glands, including a cross section of one kidney. The adrenal glands produce a variety of endocrine hormones involved in metabolism. The kidneys filter impurities such as excess salt, urea and ammonium, and are also vitally involved in maintaining proper fluid balances within the body. Kidney stones or renal calculi are formed in the calyx, pelvis, ureters or urinary bladder.
Figure A-16
The urinary system. Urine is collected in the kidneys and drained via the ureters into the bladder, where it is held until it can be passed out of the body via the urethra.
Figure A-18
Anatomy of the brain. The pia mater, arachnoid and dura mater are three membranes that surround and protect the brain within the skull. Cerebrospinal fluid circulates within the subarachnoid space and the ventricles of the brain, providing further cushioning. The main, folded part of the brain is the cerebrum and is mainly involved in perception and thought processs. The lower reddish portion is the cerebrum which coordinates balance and coordination. The brain stem, at the top of the spinal cord, controls basic functions such as breathing and heart rate.
Figure A-19
The ear. Sound is focussed by the outer ear and carried inside by the auditory canal. It then causes vibration of the outer eardrum. These vibrations are amplified and modified by the ossicles (three bones called the incus, or anvil, the malleus, or hammer, and the stapes, or stirrup), and then transferred to the inner eardrum. The sound vibrations then pass into the cochlea where they are converted into nerve signals that correspond to different frequencies and amplitudes of sound. The semicircular canals, though part of the inner ear structure, are involved in balance and spatial orientation rather than hearing.
Figure A-20
The eye. Light passes through the cornea, which provides both structure to the eye and some initial focussing. The amount of light entering the inside of the eye is controlled by the iris, which contracts or expands in order to change the pupil opening. Further focussing is done by the lens, whose shape and therefore focal distance can be modified by the ciliary muscles. The focussed light falls on the retina, where rod and cone cells convert the light into nerve signals corresponding to color and intensity. These signals are collected into the optic nerve and passed to the brain. The sclera, aqueous humor and vitreous humor provide the physical structure of the eye.
Health Technology Management
The following material is from a WHO document that is no longer available; however, the information is still valid and useful.
Health technology management units – Introduction
Organization and execution of all activities of health technology management (HTM) require skilled staff on both a technical and a managerial level. In a clinical engineering department, the technical personnel usually consist of technicians and clinical or biomedical engineers.
Biomedical or clinical engineers are educated in general engineering principles, the physical and biological sciences and their application to medical technology. Technicians, on the other hand, receive technical training with a primary focus on medical equipment maintenance. Alternatively, particularly in countries with fewer specialized training programmes, engineers and technicians may be trained in a related field (such as industrial engineering or electrical technology) and have taken certificate courses, received training or completed an apprenticeship enabling them to work in the area of medical equipment.
The engineering management personnel provide leadership. They set department policies, provide budget recommendations, supervise technical staff, arrange for training, set priorities for the department activities and develop and administer the overall programmes. The background of those in this position would include a biomedical or clinical engineering degree or similar, and familiarity with the health care environment and health care technology or a combination of business and technical training.
Health technology management should be carried out on all levels of health care and ideally should be coordinated by a designated health technology management unit within the ministry of health that dictates policies on planning of medical equipment allocation, development of technical specifications for procurement purposes, application/user training or other related elements. It should relate to other government agencies like the regulatory agency or the health technology assessment or similar units in the ministry of health.
Decision-makers can consult national centres for health technology for information on a host of issues including:
- Medical equipment per facility, technical specifications, procurement best practices, maintenance procedures, content of user training courses, and steps required for certificate of need authorization.
Health technology management teams on all facility and administrative levels need to work together to ensure coordination and supervision across the entire system.
Procurement of health technologies is an indispensable element to ensure availability of products in health care service delivery. It can be defined as "the acquisition of property, plant and/or equipment, goods, works or services through purchase, hire, lease, rental or exchange" and is taken to include "all actions from planning and forecasting, identification of needs, sourcing and solicitation of offers, evaluation of offers, review and award of contracts, contracting and all phases of contract administration until delivery of the goods, the end of a contract, or the useful life of an asset".
In summary, standard procurement procedures comprise technology evaluation, planning and needs assessment, the actual procurement of the technology, installation, commissioning, and monitoring (Fig. 3.4-6).
Poor practices in procurement can lead to substandard provision or performance of health technology. Effective health technology procurement practice, on the other hand, can lead to safe, equitable and quality health care, and all parties involved can obtain the following benefits:
- procurement staff gain by carrying out clear and accountable work done to internationally accepted standards;
- funding agencies can trust that quality goods are being procured at the right price;
- health service professionals obtain safe quality materials and tools that comply with accepted standards; and
- most importantly, at the end of the process, patients can receive appropriate and effective health care treatment, if the medical devices purchased are handled effectively by the health care workers.
Good practices include transparency, good governance, the most economically advantageous for the equipment acquired – not necessarily the lowest price obtained through tender, but a good quality product that satisfies the need of the organization and of the final users; achieving timely delivery and handover; defining satisfactory and well-defined terms for delivery, installation, commissioning, training, payment and warranty; obtaining satisfactory after-sales service; and generating greater interest from the suppliers and manufacturers in submitting offers in the future.
Medical devices inventory management
Health technologies and in particular medical devices are essential in the delivery of quality health care as they enable health care providers to diagnose, treat, monitor and provide therapy to patients within an appropriate environment of care.
Quality management of medical devices helps ensure that these services are provided in a safe and effective way. Here, the medical devices inventory plays a vital role. Inventory management’s main tasks are to record the purchase, receipt, retirement and discarding of equipment. Moreover, once properly established, a medical device inventory is a powerful tool in the clinical engineering department and the health care facility as a whole, as it is used as input for various areas in the health care management cycle. It serves as the foundation for moving forward within the health technology management system and for ensuring safe and effective medical equipment on many levels. It helps to develop budgets for capital purchases, maintenance and running costs; it helps to build and support an effective clinical engineering department by allowing for workshop planning, hiring and training of technical support staff and establishing and maintaining service contracts; it helps to support an effective medical equipment management programme, including planning preventive maintenance activities and tracking work orders; and it helps to plan the necessary stock of spare parts and consumables.
Furthermore, developing replacement and disposal policies, developing purchasing and donations goals, analyzing facility risk and mitigation, emergency and disaster planning, and equipment needs assessments are all supported by the existence of a medical devices inventory.
Inventory management can be classified into three stages: First, the inventory of all medical devices has to be compiled. Here, accessories, consumables and spare parts inventories should be directly correlated with the main medical equipment inventory. Second, the inventory needs to be updated whenever there is any change. Third, an annual audit needs to be performed. The health care facility decides on the level of detail of data to be included in its inventory in order to satisfy its own requirements and according to its own capabilities.
Medical device maintenance – Introduction
Medical devices are assets that directly affect human lives. Some of them are considerable investments for which not only the procurement costs have to be taken into account but also the costs for operation, maintenance and consumables, which are often much higher than the initial costs. The maintenance costs especially, are often underestimated. In order to keep the medical equipment in a health care institution reliable, safe and available for use when it is needed for diagnostic procedures, therapy, treatments and monitoring of patients, it is essential for a health care facility – regardless of its size – to have a well-planned and well-managed maintenance programme. Such a programme also prolongs the useful life of the equipment and thereby minimizes the cost of equipment ownership.
A maintenance programme includes the following two types of procedures:
- Procedures for performance and safety inspection and preventive maintenance (IPM):
- Performance inspections ensure that equipment is operating correctly, and safety inspections ensure the equipment is safe for both patients and operators. Preventive maintenance aims to extend the life of the equipment and reduce failure rates. Additionally, some hidden problems may be discovered during a scheduled inspection. However, performing inspections of equipment only ensures that the device is in good operating condition at the time of inspection and cannot eliminate the possibility of failure during future use; the nature of most electrical and mechanical components is that they can potentially fail at any time.
- Procedures for corrective maintenance (CM):
- Corrective maintenance restores the function of a failed device and allows it to be put back into service. Identification of a device failure usually occurs when a device user has reported a problem with the device or when a technician finds that a device is not performing as expected during IPM. After completion of repair, it is essential to conduct a performance and safety inspection, and in some cases a re-calibration may be required.
To plan, manage, and implement an effective medical equipment maintenance programme is a complex task. It is important to have a well-functioning clinical engineering department in place, which needs competent staff such as experienced biomedical engineers and well-trained equipment technicians.
Further material on HTM from the WHO can be found at this site.
Medical device history
https://medicine.yale.edu/news/yale-medicine-magazine/article/medical-devices-and-technology-across-the-years/
https://www.mddionline.com/rd/earliest-medical-device-innovators
https://www.glenmedsolutions.com/2018/07/12/5-greatest-medical-equipment-inventions-in-history/
https://artsandculture.google.com/story/lifeblood-the-history-and-future-of-medical-equipment-technoseum/fAXBPxkFvBidIg?hl=en
- Click to view external links