Start
TFP is a 3 year old girl who was admitted to the Emergency Department of her local hospital in a coma after two days when she had a fever and had been refusing food. She has a history of muscle weakness, and at the time of her admission her muscle tone was very poor - she was described as being "floppy". A blood sample taken on admission gave the following results:
TFP |
reference range (after
overnight fast) |
|
| glucose (mmol /L) | 2.1 mmol /L |
3.5 - 5.5 |
| non-esterified fatty acids (mmol /L) | 2.5 mmol /L |
0.8 - 1.4 |
| ketone bodies (mmol /L) | not detectable |
1.0 - 2.5 |
| pH | 7.40 |
7.35 - 7.45 |
| bicarbonate | 23 |
21 - 25 |
| insulin (mU /L) | 3.5 |
4 - 8 |
What do you think was the cause of her coma?
She is profoundly hypoglycaemic, and this is the immediate cause of her coma - there is no evidence of acidosis, and her plasma insulin is appropriate for her hypoglycaemia (i.e. there is no evidence to suggest that her hypoglycaemia is caused by excess insulin secretion).
What is interesting is that she has a high plasma concentration of non-esterified fatty acids, but no ketone bodies are detectable. This suggests a possible defect in either the synthesis of ketone bodies or the oxidation of fatty acids. Her history of muscle weakness suggests that her underlying problem may be an inability to oxidise fatty acids, which form a major fuel for skeletal muscle. (See also the problem on muscle weakness, heart failure and profound hypoglycaemia in a young girl ).
She recovered after an intravenous infusion of glucose, although her muscle tone and muscle strength were still poor. Again this is suggestive of a defect in fatty acid oxidation.
The pathway of fatty acid oxidation was elucidated at the beginning of the 20th century, although it was not until the middle of the century that the early deductions were confirmed experimentally.
 In 1904 Knoop reported the results of injecting dogs with two compounds: phenylpropionic acid and phenylbutyric acid. These are both (very) short chain fatty acids, with a phenyl group in place of the terminal methyl group. Phenylpropionic acid is a phenyl-labelled odd-carbon fatty acid ( 3-carbons), while phenylbutyric acid is a phenyl-labelled even-carbon fatty acid (4 carbons).
In 1904 Knoop reported the results of injecting dogs with two compounds: phenylpropionic acid and phenylbutyric acid. These are both (very) short chain fatty acids, with a phenyl group in place of the terminal methyl group. Phenylpropionic acid is a phenyl-labelled odd-carbon fatty acid ( 3-carbons), while phenylbutyric acid is a phenyl-labelled even-carbon fatty acid (4 carbons).
The end-products of oxidation of these two fatty acids were excreted as glycine and glucuronic acid conjugates; hydrolysis of the conjugates with hydrochloric acid showed that:
- phenylpropionic acid was excreted as benzoic acid conjugates
- phenylbutyric acid was excreted as phenylacetic acid conjugates
What conclusions can you draw from these results?
If oxidation of fatty acids occurred as a result of oxidation of one carbon atom at a time to carbon dioxide then you would expect both odd-carbon and even-carbon phenyl-labelled fatty acids to yield the same product, benzoic acid. However, this is not what was observed. The conclusion that Knoop drew was that two carbon atoms are removed at a time in fatty acid oxidation.
Why do you think the term beta-oxidation was adopted for this oxidative removal of two carbon atoms at a time?
 Remember that in this (old-fashioned) nomenclature, the alpha-carbon is the carbon atom to which the functional group for which the compound is named. In this case, the functional group is the carboxyl group. In correct systematic modern nomenclature the alpha-carbon would be carbon-2, since carbon-1 is the carboxyl carbon.
Remember that in this (old-fashioned) nomenclature, the alpha-carbon is the carbon atom to which the functional group for which the compound is named. In this case, the functional group is the carboxyl group. In correct systematic modern nomenclature the alpha-carbon would be carbon-2, since carbon-1 is the carboxyl carbon.
Counting from the alpha carbon, the atoms are then named in (Greek) alphabetical order. We have already seen in the exercise on fats and oils - are all fats the same? that this nomenclature is still useful, because human beings have enzymes that can introduce additional carbon-carbon double bonds between an existing double bond and the carboxyl group, but not between an existing double bond and the methyl group (the omega-carbon). This means that the position of the first double bond from the omega-carbon determines which family of unsaturated fatty acids a given compound belongs to.
 Rats were fed [14C-U]palmitate (the 16-carbon saturated fatty acid, labelled with 14C in all carbon atoms) and phenyl aminobutyrate, which is excreted as the N-acetylderivative.
Rats were fed [14C-U]palmitate (the 16-carbon saturated fatty acid, labelled with 14C in all carbon atoms) and phenyl aminobutyrate, which is excreted as the N-acetylderivative.
The N-acetyl phenyl aminobutyrate was recovered from the rat urine, hydrolysed by heating in hydrochloric acid and the acetate was separated by chromatography. It was found that the acetate was labelled with 14C.
What conclusions can you draw from this observation?
 This confirms that fatty acid oxidation occurs by stepwise removal of 2-carbon units, as acetate (or an active acetyl derivative).
This confirms that fatty acid oxidation occurs by stepwise removal of 2-carbon units, as acetate (or an active acetyl derivative).
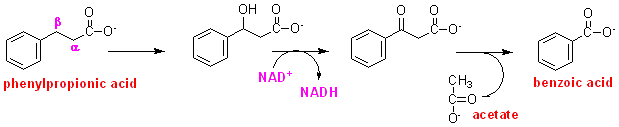
 In studies to determine how beta-oxidation occurs, dogs were injected with a relatively large amount of phenylpropionic acid; most of the dose was recovered in the urine as conjugates of benzoic acid, but trace amounts of phenylhydroxypropionic acid and phenyloxopropionic acid were also recovered, as shown on the right.
In studies to determine how beta-oxidation occurs, dogs were injected with a relatively large amount of phenylpropionic acid; most of the dose was recovered in the urine as conjugates of benzoic acid, but trace amounts of phenylhydroxypropionic acid and phenyloxopropionic acid were also recovered, as shown on the right.
What conclusions can you draw from these results?
From these results we can begin to draw up a pathway for fatty acid oxidation, beginning with introduction of a hydroxyl group on the beta-carbon, followed by oxidation of the hydroxyl group to an oxo group (by transfer of 2H onto a carrier of some kind (in fact we now know that this carrier is NAD). Cleavage of the terminal acetyl group will leave a new carboxyl group at what was the beta-carbon:
Can you draw out a sequence of reactions for the oxidation of palmitate from the information you have gained so far?
 By analogy with the sequence of reactions shown above for the oxidation of phenylpropionic acid, it is possible to draw up a sequence of reactions involving introduction of a hydroxyl group at the beta-carbon of palmitate, oxidation to an oxo group, then cleavage to remove acetate and leave the 14-carbon fatty acid, myristate.
By analogy with the sequence of reactions shown above for the oxidation of phenylpropionic acid, it is possible to draw up a sequence of reactions involving introduction of a hydroxyl group at the beta-carbon of palmitate, oxidation to an oxo group, then cleavage to remove acetate and leave the 14-carbon fatty acid, myristate.
This 14-carbon fatty acid is presumably available to undergo the same sequence of reactions, until at the final stage the 4-carbon fatty acid (butyrate) yields two mol of acetate.
This sequence of reactions is shown on the right.
We now need to consider the source of the hydroxyl group that is introduced into the fatty acid at each cycle of reactions.
In the next set of experiments isolated liver cells were incubated with phenylpropionic acid:
a) in the presence of 18O2
b) in the presence of water labelled with 18O (H218O)
No label was incorporated into benzoate or acetate when the label was in oxygen gas; however, when the cells were incubated with 18O labelled water there was incorporation of label into benzoate at the same isotopic enrichment as that in the water in the incubation medium.
How do you think the oxygen can be incorporated from water?
It is likely that oxygen from water can be incorporated by adding water across a carbon-carbon double bond:

How might you test the hypothesis that the first reaction in beta-oxidation is dehydrogenation to form a carbon-carbon double bond, so that water can be incorporated across it to form a hydroxyl group?

 Phenylpropionate was synthesised with 3H at the alpha- and beta-carbons, and was used as a substrate for isolated liver cells. After 30 min the reaction was stopped by addition of trichloroacetic acid, and precipitated protein was centrifuged out. Half the supernatant was subjected to liquid chromatography to separate and identify soluble compounds, and measure the radioactivity in them. The other half of the supernatant was subjected to micro-distillation to separate the water . The results were as follows (3H dpm per 100 mg protein in the incubation, mean ± sd for 3 x replicate incubations:
Phenylpropionate was synthesised with 3H at the alpha- and beta-carbons, and was used as a substrate for isolated liver cells. After 30 min the reaction was stopped by addition of trichloroacetic acid, and precipitated protein was centrifuged out. Half the supernatant was subjected to liquid chromatography to separate and identify soluble compounds, and measure the radioactivity in them. The other half of the supernatant was subjected to micro-distillation to separate the water . The results were as follows (3H dpm per 100 mg protein in the incubation, mean ± sd for 3 x replicate incubations:
unincubated control |
after 30 min incubation |
|
| phenylpropionate | 5200 ± 85 |
1120 ± 97 |
| benzoate | not detectable |
1011 ± 96 |
| acetate | not detectable |
1099 ± 101 |
| water | not detectable |
2100 ± 136 |
What conclusions can you draw from these results?
 These results suggest that the first reaction is indeed a dehydrogenation reaction, and that ultimately the hydrogen removed from the phenylpropionate is used to reduce oxygen to water. Although you do not have the information to deduce it, this dehydrogenation reaction occurs by reduction of the flavin coenzyme, FAD. The resultant FADH2 is then reoxidised in the mitochondrial electron transport chain, ultimately reducing oxygen to water.
These results suggest that the first reaction is indeed a dehydrogenation reaction, and that ultimately the hydrogen removed from the phenylpropionate is used to reduce oxygen to water. Although you do not have the information to deduce it, this dehydrogenation reaction occurs by reduction of the flavin coenzyme, FAD. The resultant FADH2 is then reoxidised in the mitochondrial electron transport chain, ultimately reducing oxygen to water.
We now know that the fatty acids do not undergo oxidation as free fatty acids, but esterified to coenzyme A, an the acetate is not released as acetate, but as acetyl CoA.
From the information you have gathered to date, can you write out the full sequence of reactions in the first cycle of beta-oxidation of palmitate (C16:0, as palmitoyl CoA) to yield myristate (C14:0, as myristyl CoA) and acetate (as acetyl CoA)?
The full sequence of reactions is as follows:

The product of each cycle of beta-oxidation then undergoes the same sequence of reactions until the final product is butyryl CoA (C4:0), which is cleaved by CoASH to yield 2 mol of acetyl CoA.
What is the ATP yield for each cycle of the reactions shown above?
In each cycle of reactions 1 x FAD and 1 x NAD are reduced to NADH. Oxidation of FADH is the mitochondrial electron transport chain yields ~ 1.5 x ATP, and of oxidation of NADH yields ~2.5 x ATP, so the yield of each cycle of beta-oxidation ~4 x ATP.
What is the ATP yield for palmitate (C16:0) being oxidised to acetyl CoA?
 Complete oxidation of palmitoyl CoA to acetyl CoA requires 7 cycles of reaction, so the overall ATP yield = 7 x 4 = 28 x ATP. However, there is a requirement for 2 x ATP equivalents to form palmitoyl CoA from palmitate in the first place,
Complete oxidation of palmitoyl CoA to acetyl CoA requires 7 cycles of reaction, so the overall ATP yield = 7 x 4 = 28 x ATP. However, there is a requirement for 2 x ATP equivalents to form palmitoyl CoA from palmitate in the first place,
so the net yield is 28 - 2 = 26 x ATP.
What is the yield of ATP for palmitate undergoing complete oxidation to carbon dioxide and water?
For each acetyl CoA undergoing oxidation in the citric acid cycle there is a yield of
- 3 x NADH (=~3 x 2.5 = 7.5 ATP)
- 1 x FADH (=~1.5 x ATP)
- 1 x ATP (or GTP) formed by substrate level phosphorylation
total = 10 x ATP per acetyl CoA oxidised:
- 8 x acetyl CoA from palmitate oxidised to carbon dioxide and water = 8 x 10 = 80 ATP
- 7 cycles of beta-oxidation = 28 x ATP
- minus 2 x ATP for formation of palmitoyl CoA
total = 106 x ATP for each mol of palmitate oxidised to carbon dioxide and water
Although we know the sequence of reactions involved, none of the intermediates of beta-oxidation is found in cells or in the incubation medium after in vitro experiments.
If mitochondria are incubated with [14C-U]palmitatoyl CoA(i.e. palmitoyl CoA in which all 16 carbon atoms of the fatty acid are labelled with 14C) traces of labelled medium-chain (C14:0) and short-chain (C8:0) fatty acyl CoA are found.
What conclusions can you draw from this observation?
If none of the intermediates of beta-oxidation is released into the cell or incubation medium then it is likely that the enzymes involved are closely associated, either as a multi-enzyme complex, or adjacent to one another, in such a way that the product of one enzyme is passed immediately to the active site of the next, without leaving the enzyme surface.
Studies of the long-chain hydroxyacyl CoA dehydrogenase have shown that three of the enzymes of beta-oxidation are found in a single trifunctional protein. There is a large subunit that catalyses hydroxylation of the unsaturated fatty acyl CoA and dehydrogenation of the hydroxy-derivative, and a small subunit that catalyses the cleavage by CoASH. THis trifunctional protein and the fatty acyl CoA dehydrogenase are bound to the matrix face of the inner mitochondrial membrane.
The fact that traces of labelled medium- and short-chain fatty acyl CoA are found after incubation with [14C-U]palmitoyl CoA suggests that there may be three sets of enzymes involved in beta-oxidation, with specificity for long, medium and short-chain fatty acyl CoA, and that the final product of each does indeed leave the membrane-bound enzyme complex and travel to the next.
We can now return to consider TFP. Among other tests that were carried out while she was in hospital, a urine sample was subjected to high pressure liquid chromatography, and a number of abnormal carnitine esters of organic acids (especially 2-enoylpalmitate and 3-hydroxypalmitate, see the overview of beta-oxidation above) were found. Genetic analysis showed that she had a deletion mutation in the gene for the long-chain fatty acid trifunctional protein.
Why do you think she suffered from muscle weakness and fasting hypoketotic coma?
Fatty acids are a major fuel for muscle, and if she cannot oxidise them it is likely that she ill suffer from muscle weakness and myopathy.
In the fasting state, the ketone bodies (beta-hydroxybutyrate and acetoacetate) are synthesised from acetyl CoA, which in turn is formed by beta-oxidation of fatty acids. These fatty acids come from adipose tissue triacylglycerol stores, and are mainly long-chain fatty acids (C16:0 and C18:0). If beta-oxidation of long-chain fatty acids is impaired, she will be unable to synthesise ketone bodies from their adipose tissue triacylglycerol reserves.
What long-term treatment might be appropriate for her?
Apart from preventing prolonged fasting (i.e. providing frequent small meals, and perhaps supplementary feeding via a naso-gastric tube through the night) it would be possible to feed her triacylglycerols containing medium-chain fatty acids, since if she lack the long-chain fatty acid trifunctional protein she should still have adequate activity of the medium- and short-chain multi-enzyme complexes.
Key points from this exercise:
- Fatty acids (as fatty acyl CoA esters) undergo oxidation at the beta-carbon. The steps are:
- dehydrogenation to form a carbon-carbon dounble bond between teh alpha and beta-carbons, lined to reduction of FAD
- addition of water across te double bond to yield a hydroxyl group at the beta-carbon
- oxidation of the hydroxyl group to an oxo group, linked to the reduction of NAD
- cleavage by formation of a thiester to CoA at the new oxo-group, yilding acetyl CoA and a fatty acyl CoA that is two carbons shorter than the staryting compound.
- Each cycle of beta-oxidation yields ~ 4 x ATP, but there is an initial cost of 2 x ATP to form palmitoyl CoA from palmitate
- complete oxiadtion of palmitate to acetyl CoA yields 26 x ATP
- complete oxiadtion of palmitate to carbon dioxide and water yields 106 x ATP
- The intermediates of the beta-oxidation pathway are not detected in tissues, suggesting that the enzymes are closely associated in a multi-enzyme complex. However, traces of medium- and short-chain fatty acyl CoA are found when loing-chain fatty acyl CoA is being oxidised, suggesting that there are three separate sets of nzymes, with specificity for long-, medium- and short-chain fatty acyl CoA.
- In the long-chain hydroxyacyl CoA dehydrogenase three of the enzymes of beta-oxidation are found in a single trifunctional protein. A large subunit catalyses hydroxylation of the unsaturated fatty acyl CoA and dehydrogenation of the hydroxy-derivative, and a small subunit catalyses the cleavage by CoASH. THis trifunctional protein and the fatty acyl CoA dehydrogenase are bound to the matrix face of the inner mitochondrial membrane.
- Children who have a genetic defect of the long-chain trifunctional protein suffer from hypoketotic coma in fasting because they are unbable to oxidised the long-chain fatty acids released from adipose tissue to form acetyl CoA as a substrate for ketone body synthesis. They can metabolism medium- and short-chain fatty acids normally.