Start
Part 1
HG gave a small dinner party, and in honour of one of the guests who was Jamaican, prepared a traditional Jamaican dish, ackee with rice and salt fish, as well as a number of other dishes. Unfortunately, she had used unripe ackee fruit. Only two of the guests ate any of ackee dish, and about an hour after the meal both became unconscious. An ambulance was called and they were taken to hospital. Emergency measurement of their blood glucose showed that it was dangerously low: 1.9 mmol /L in one case and 2.1 mmol /L in the other.
What is the normal range of blood glucose?
What emergency treatment should they received?
The normal range of blood glucose is 3.5 - 5.5 mmol /L, but may rise to 8 mmol /L after a meal. (You will consider problems associated with hyperglycaemia, concentations of glucose considerably higher than normal, in later exercises)
The obvious emergency treatment would be intravenous glucose.
(We will come back to investigate how unripe ackee causes severe hypoglycaemia later in this exercise)
Why does a very low blood glucose concentration lead to loss of consciousness?
The brain is more or less completely reliant on glucose as its metabolic fuel, except in prolonged starvation, and, as can be seen from figure 1 below, the brain accounts for about 20% of whole body resting energy expenditure. Most of this energy expenditure is involved in transporting sodium and potassium ions across nerve membranes, against their concentration gradient, to maintain electrical activity. Figure 2 shows that the sodium pump (in the brain and all other tissues) accounts for about 22% of whole body resting energy expenditure.
Figure 1: Percentage of resting energy expenditure by different organs
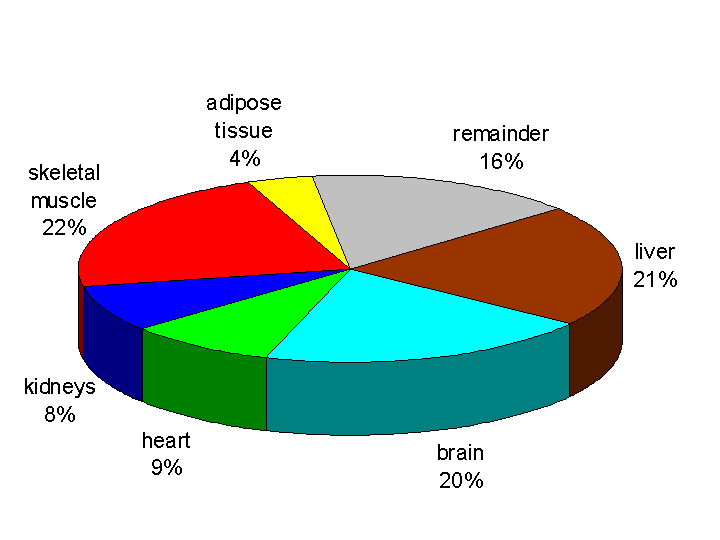
Figure 2: Percentage of resting energy expenditure in different processes

How can sodium and potassium ions be transported across cell membranes against their concentration gradient?
To transport ions against their concentration gradient requires an input of energy.
Here we need to introduce the compound adenosine triphosphate (ATP), which has a high free energy of hydrolysis to yield adenosine disphosphate (ADP) and inorganic phosphate. In addition to its role in ion transport, you will see later in this exercise how ATP can be used to drive endothermic reactions in the thermodynamically unfavoured direction, and how it is involved in muscle contraction and hence the performance of physical work.
You will see in later exercises how the phosphorylation of ADP back to ATP is linked to the oxidation of metabolic fuels, so that it acts as an intermediate between the oxidative pathways that are energy yielding and the various processes that are energy requiring.

What happens in sodium / potassium transport is that the carrier in the membrane can exist in two conformations:
- With ATP bound it faces inwards, and binds 3 x sodium ions. The binding of sodium ions leads to phosphorylation of the carrier, release of ADP and a conformational switch so that it now faces outwards and expels sodium ions into the extracellullar fluid.
- Two potassium ions now bind to the outward facing transporter, causing dephosphorylation. ATP binds to the dephosphorylated transporter, causing it to flip to face inwards again, expelling potassium ions into the cytosol, and ready to bind sodium ions again.
How can ATP be used to drive endothermic reactions in the thermodynamically unfavoured direction?
An endothermic reaction requires an input of energy if it is to proceed in the thermodynamically unfavoured direction. In the laboratory this is often achieved by heating the reaction mixture (often to a very high temperature), something that is obviously not possible in the body. There are two ways in which ATP can be used - both overall result in the hydrolysis of ATP to ADP and inorganic phosphate:
- phosphorylation of the enzyme as an intermediate stage of the reaction, so creating a strongly charged group at the active site of the enzyme (in a later exercise you will see how enzymes catalyse reactions by lowering the activation energy)
- phosphorylation of the substrate, so creating a group that can readily be displaced.

How is ATP involved in muscle contraction?
 The key proteins in muscle are myosin, a fibrous protein that has ATPase activity in the light chains that make up the head region of the protein, and actin, a globular protein. Molecules of actin are arranged along a filament of tropomyosin (interspersed with molecules of troponin, a calcium-binding regulatory protein). The head regions of myosin are associated with actin, and the process of contraction involves the head regions moving along the actin chain to become associated with another actin molecule, and the tail regions sliding over one another.
The key proteins in muscle are myosin, a fibrous protein that has ATPase activity in the light chains that make up the head region of the protein, and actin, a globular protein. Molecules of actin are arranged along a filament of tropomyosin (interspersed with molecules of troponin, a calcium-binding regulatory protein). The head regions of myosin are associated with actin, and the process of contraction involves the head regions moving along the actin chain to become associated with another actin molecule, and the tail regions sliding over one another.
Myosin that is tightly bound to actin has ADP bound. Replacement of this ADP by ATP loosens the binding to actin; hydrolysis of the ATP to ADP and phosphate loosens it further, and release of phosphate (leaving ADP bound to myosin) causes the myosin fibres to slide over each other and the head regions to be associated with actin molecules further along the chain.
Part 2
HG gave a small dinner party
HG gave a small dinner party, and in honour of one of the guests who was Jamaican, prepared a traditional Jamaican dish, ackee with rice and salt fish, as well as a number of other dishes. Unfortunately, she had used unripe ackee fruit. Only two of the guests ate any of ackee dish, and about an hour after the meal both became unconscious. An ambulance was called and they were taken to hospital. Emergency measurement of their blood glucose showed that it was dangerously low: 1.9 mmol /L in one case and 2.1 mmol /L in the other.
 Unripe ackee contains an unusual amino acid, called hypoglycin, because of its effect in causing (potentially fatal) hypoglycaemia. The amount of hypoglycin decreases as the fruit ripens, so that ripe ackee is not toxic. Hypoglycin is metabolised to a coenzyme A derivative that does not undergo further metabolism. (You do not have to know the chemistry involved in the reaction shown on the right, but you will come across the reactions involved in the second step when you consider glucose metabolism and the citric acid cycle).
Unripe ackee contains an unusual amino acid, called hypoglycin, because of its effect in causing (potentially fatal) hypoglycaemia. The amount of hypoglycin decreases as the fruit ripens, so that ripe ackee is not toxic. Hypoglycin is metabolised to a coenzyme A derivative that does not undergo further metabolism. (You do not have to know the chemistry involved in the reaction shown on the right, but you will come across the reactions involved in the second step when you consider glucose metabolism and the citric acid cycle).
If you are interested to learn more about ackee, follow this link
What is coenzyme A, and how is it involved in metabolism?
 Coenzyme A has the structure shown on the left. It is synthesised from the vitamin pantothenic acid and cysteamine, the amine derived from the amino acid cysteine. You do not need to know its structure. The important point is that it forms thioesters with fatty acids, and is these fatty acyl CoA esters that undergo oxidation.
Coenzyme A has the structure shown on the left. It is synthesised from the vitamin pantothenic acid and cysteamine, the amine derived from the amino acid cysteine. You do not need to know its structure. The important point is that it forms thioesters with fatty acids, and is these fatty acyl CoA esters that undergo oxidation.
Because it has a free sulphydryl group, it is conventional to show free coenzyme A as CoA, and the fatty acyl CoA ester as fatty acyl CoA.
The metabolism of glucose also involves coenzyme A - the three carbon compound pyruvate undergoes an oxidative decarboxylation reaction similar to that shown above, to yield acetyl CoA, which then enters the citric acid cycle for metabolism to carbon dioxide and water (yielding a considerable amount of ATP in the process).
How do you think it is that a relatively small amount of hypoglycin can cause such profound hypoglycaemia?
Do you think there is a large or small amount of coenzyme A in cells?
There is a relatively small amount of coenzyme A in cells. The same is true of ATP - the total amount of (ATP + ADP) in the body is only about 5 grams, but the total amount of ATP used each day is approximately equal to body weight - say 70 kg. As fast as ATP is converted to ADP and phosphate, in performing chemical and physical work, so ADP is rephosphorylated back to ATP, linked to the oxidation of metabolic fuels.
Hypoglycin forms a coenzyme A derivative that does not undergo further metabolism, so each molecule of hypoglycin will effectively remove a molecule of coenzyme A from the pool in tissues. This will result in severe impairment of the metabolism of both fatty acids and glucose. The only source of ATP formation will be the relatively small amount that is formed in the metabolism of glucose to pyruvate. Therefore, in an effort to produce enough ATP to maintain life, carbohydrate reserves will be severely depleted, leading to profound hypoglycaemia.
Key points from this exercise:
- The brain is more or less completely reliant on glucose as its metabolic fuel, except in prolonged starvation, and it accounts for about 20% of whole body resting energy expenditure. Most of this energy expenditure is involved in transporting sodium and potassium ions across nerve membranes, against their concentration gradient, to maintain electrical activity.
- ATP is involved in three main areas:
- active transport of ions (and metabolites) against their concentration gradient
- forcing endothermic reactions in the thermodynamically unfavoured direction
- providing the driving force for muscle contraction
- Coenzyme A is involved in metabolism as a carrier of fatty acids, forming fatty acyl CoA thioesters
- Like other coenzymes, coenzyme A is present in very small amounts in the cell, but turns over rapidly